ABOUT NAVEL
“Patients are our number-one priority”
|
The current treatment for aortic valve replacements is to perform a transcatheter aortic valve replacement (TAVR). TAVR is a minimally invasive procedure that accesses the aorta through the femoral artery and replaces traditional open-heart surgery. The current issues regarding femoral insertion include difficulty accessing and maintaining an ideal entry location and angle to the femoral artery, high push force when inserting the needle or sheath, and the kinking of the sheath because of large insertion angles. These issues are often exacerbated by obesity. We are attempting to fix these issues with our device using ultrasound guided augmented reality and a needle driver device to find and maintain the best angle of entry. In turn, this will lower the push force. Our device also uses shallower angles of entry which avoids later kinking of the sheath |
| Needs Statement: |
In cardiac research, developing a femoral access tool for an aortic sheath and guide wire is an inherent clinical need. The target population is for patients who suffer from vascular complications such as stenosis during trans femoral aortic valve replacement (TAVR). As biomedical engineers, utilizing a desired angle of 30 degrees or less is ideal in targeting the femoral artery to correctly implant the aortic sheath.
Competitor Analysis:
Advantages to our Device:
- Software allows maximum control over needle guidance parameters
- Multiple Needle Drivers allow for tailored procedure
- Very cheap components (less than $500 w/o ultrasound)
- Can connect to any ultrasound machine with VGA Out
- Needle driver designed with Seldinger needle insertion technique in mind
- Cheap components make for an ideal training model for medical student
Disadvantages to our Device:
- Software adds additional complexity
- Capture card connection requires multiple steps and can be unstable
- Lack of needle data within tissue
- Multiple separate system components
Market Strategy:
——————————————————————————————-
Target Market
- Our device is made for physicians performing TAVR procedures and those training for future TAVR procedures
- Approximately 100,000 – 120,000 TAVR procedures are performed a year in the U.S.
Financial Projections
- Software: $2,000
- One time purchase including an ultrasound machine and probe, laptop, and augmented reality software
- Single-Use Needle Driver Device: $100
- Multiple time purchase including a Single-Use Needle Driver Device with preset angle
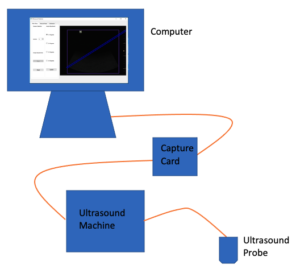
Design Overview of Ultrasound Probe

Mind Ray DP-50 Ultrasound Transducer